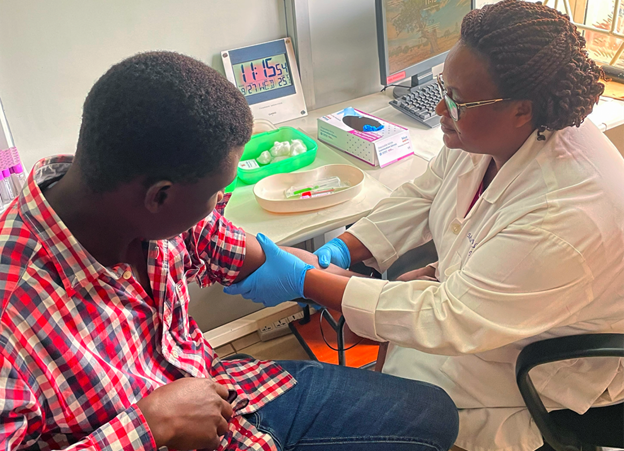
Clinical Trial Preparation Staff at Makerere University Walter Reed Project preparing for Sabin’s Phase 2 Marburg vaccine clinical trial
Exciting News for Vaccine Development
WASHINGTON, Oct. 19, 2023 (GLOBE NEWSWIRE) — The Sabin Vaccine Institute has launched a Phase 2 clinical trial for its vaccine candidate against the lethal Marburg virus. Healthy volunteers received the single-dose vaccine at Makerere University Walter Reed Project in Uganda, marking a significant milestone in the fight against this deadly disease.
What is Marburg Virus?
Marburg virus is a highly infectious virus that causes severe hemorrhagic fever in humans and non-human primates. The virus is transmitted to people from fruit bats and spreads through human-to-human transmission. There is currently no specific treatment or vaccine available for Marburg virus disease, making it a major public health concern in regions where it is endemic.
Phase 2 Clinical Trial
The Phase 2 clinical trial of Sabin’s Marburg vaccine aims to evaluate the safety and efficacy of the vaccine in a larger group of participants. The vaccine has shown promising results in preclinical studies, triggering a strong immune response against the virus. By advancing to Phase 2, researchers hope to gather more data on the vaccine’s effectiveness and safety profile, bringing them closer to potential licensure and widespread use.
Impact on Public Health
If successful, the development of a safe and effective Marburg vaccine could have a significant impact on public health, especially in regions where the virus is endemic. Vaccination programs could help prevent future outbreaks and reduce the burden of disease on affected communities. The global health community is eagerly awaiting the results of this Phase 2 clinical trial, as it could pave the way for the introduction of a much-needed tool in the fight against Marburg virus.
The World’s Response
The initiation of Sabin’s Phase 2 clinical trial for the Marburg vaccine in Uganda has garnered attention from around the world. Health organizations, policymakers, and researchers are closely following the progress of this trial, recognizing its potential to address a critical gap in infectious disease prevention and control. The global impact of a successful Marburg vaccine could extend beyond Uganda, benefiting populations at risk of the virus in other parts of the world.
How It Affects You
As a member of the global community, the development of a Marburg vaccine could have direct implications for your health and well-being. If the vaccine proves to be safe and effective, it may become a recommended part of routine vaccinations, particularly for individuals at high risk of exposure to the virus. By reducing the spread of Marburg virus, a successful vaccine could protect you and your loved ones from this deadly disease, enhancing overall public health worldwide.
Conclusion
In conclusion, the initiation of Sabin’s Phase 2 clinical trial for the Marburg vaccine represents a significant step forward in the fight against this highly infectious and deadly virus. The outcome of this trial has the potential to impact public health on a global scale, offering hope for a future where Marburg virus is no longer a threat to vulnerable populations. As we await the results of this groundbreaking research, we can look forward to a world where infectious diseases like Marburg are preventable through effective vaccination strategies.