Caribou Biosciences Announces FDA Clearance of IND Application for CB-012, an Allogeneic Anti-CLL-1 CAR-T Cell Therapy
Introduction
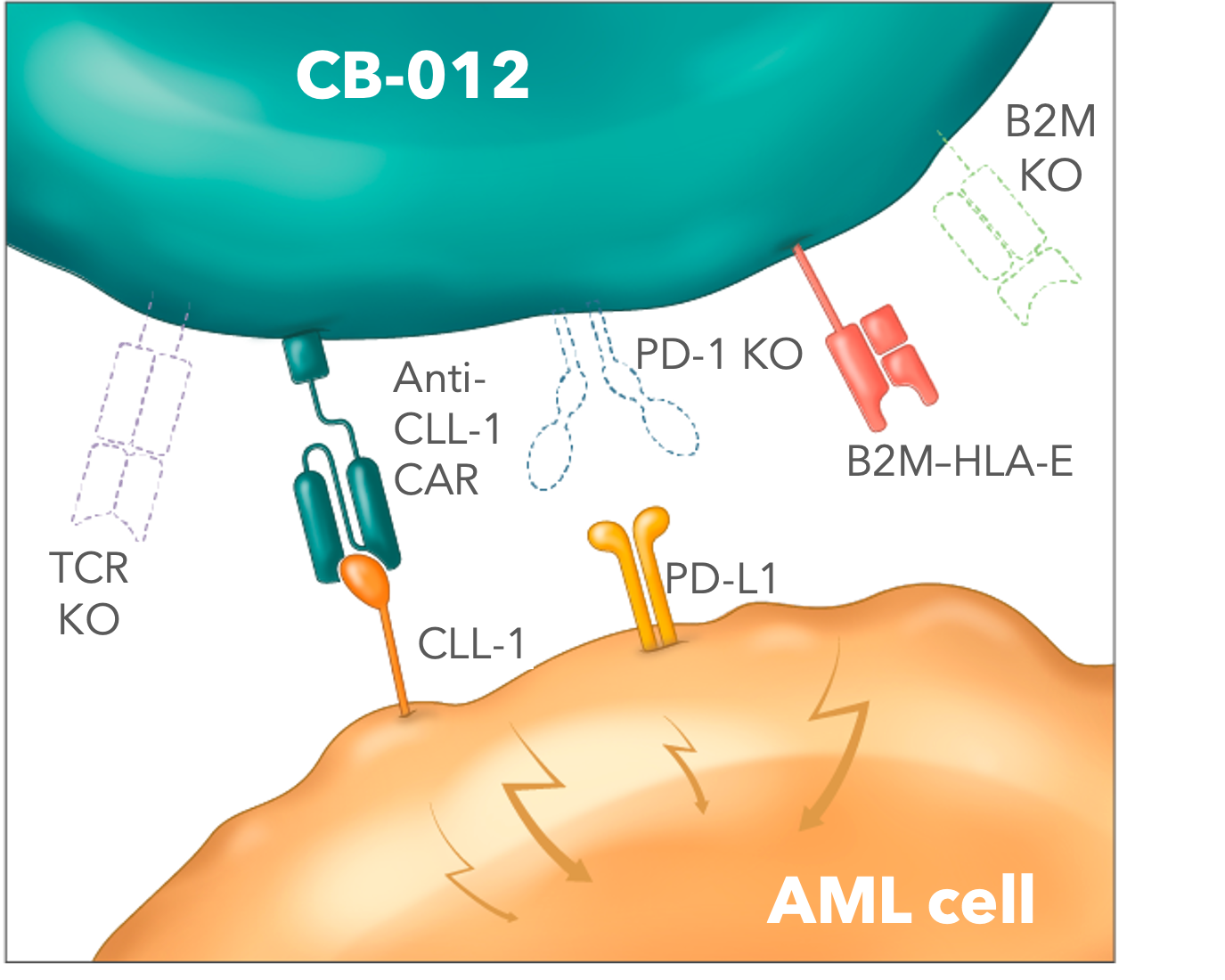
Caribou Biosciences has recently made a groundbreaking announcement regarding the FDA clearance of the Investigational New Drug (IND) application for CB-012, an allogeneic anti-CLL-1 CAR-T cell therapy. This innovative therapy combines genome editing techniques with checkpoint disruption and immune cloaking, setting it apart from existing treatments in the field. The AMpLify Phase 1 clinical trial is set to begin patient enrollment by mid-2024, marking a significant step forward in the development of allogeneic CAR-T cell therapies.
CB-012: A Game-Changer in CAR-T Cell Therapy
CB-012 represents a major advancement in the field of CAR-T cell therapy, as it is the first allogeneic treatment to incorporate both checkpoint disruption and immune cloaking mechanisms. By leveraging genome editing technologies, Caribou Biosciences has developed a therapy that has the potential to enhance anti-tumor activity while minimizing off-target effects. This dual approach holds promise for improving patient outcomes and expanding the reach of CAR-T cell therapy to a wider population.
AMpLify Phase 1 Clinical Trial
The upcoming AMpLify Phase 1 clinical trial will be a pivotal moment in the development of CB-012. This trial will evaluate the safety and efficacy of the therapy in patients with CLL-1-positive malignancies, paving the way for future clinical studies and potential regulatory approvals. By initiating patient enrollment by mid-2024, Caribou Biosciences aims to rapidly advance the development of CB-012 and bring this innovative therapy to patients in need.
Overall, the FDA clearance of the IND application for CB-012 is a significant milestone for Caribou Biosciences and the field of CAR-T cell therapy. This groundbreaking therapy has the potential to revolutionize the treatment of CLL-1-positive malignancies and improve patient outcomes. With the AMpLify Phase 1 clinical trial on the horizon, we can look forward to exciting developments in the field of allogeneic CAR-T cell therapy.
How Will This Impact Me?
As a potential future patient, the FDA clearance of CB-012 represents a promising advancement in the field of cancer therapy. This innovative CAR-T cell therapy offers new hope for individuals with CLL-1-positive malignancies, providing a potentially more effective and targeted treatment option. By incorporating checkpoint disruption and immune cloaking mechanisms, CB-012 may offer improved outcomes with reduced side effects, offering a more personalized approach to cancer treatment.
How Will This Impact the World?
The development of CB-012 has far-reaching implications for the field of oncology and CAR-T cell therapy. This innovative treatment approach has the potential to revolutionize cancer care and improve outcomes for patients worldwide. By advancing the science of genome editing and immunotherapy, Caribou Biosciences is paving the way for future advancements in precision medicine and personalized cancer therapy. The FDA clearance of CB-012 marks a significant step forward in the quest to develop novel and effective treatments for cancer.
Conclusion
The FDA clearance of the IND application for CB-012 is a major milestone in the field of CAR-T cell therapy, signaling a new era of innovation and progress in the treatment of CLL-1-positive malignancies. With the initiation of the AMpLify Phase 1 clinical trial on the horizon, we can anticipate exciting developments in the field of allogeneic CAR-T cell therapy. The potential impact of CB-012 on patient outcomes and the wider oncology landscape is significant, offering new hope for individuals battling cancer and driving advancements in precision medicine.